Chapter 5.2.3 / Global Warming Potential
Global Warming Potential
The purpose of this section is to provide information about the characteristics of greenhouse gases that contribute to their capacity to impact global warming.
Professional Development for Educators
Global Warming Potential
Why are some greenhouse gases more potent than others?
The concentration level of a greenhouse gas in the atmosphere and the Global Warming Potential (GWP) of that gas combine to determine the impact it has on global warming.
Carbon dioxide is the greenhouse gas most commonly discussed because its atmospheric concentration is increasing and the increase is largely attributed to human activity. Thus we can change behaviors to impact the concentration in the atmosphere. However, CO2 has a long life in the atmosphere, so urgency of action to reduce CO2 emissions is critical. An understanding of all greenhouse gases is necessary to appreciate the complex issue of the greenhouse effect and climate change.
Please view this video as an introduction to the topic:
Methane (CH4), an off-gas produced by decaying organic matter such as animal excrement and debris in landfills, is actually a more potent GHG than carbon dioxide. However, there is much less methane than carbon dioxide in the atmosphere and it resides in the atmosphere for a much shorter time than CO2. [On a related note: Scientists are concerned about increasing levels of atmospheric CH4 being influenced by a positive feedback mechanism as global warming is causing the release of methane trapped in arctic permafrost as it thaws due to warmer temperatures.]
The graphic below shows that there are two factors that contribut to the impact greenhouse gases have on global warming.
Global Warming Potential
Global Warming Potential (GWP) has the rather confusing definition of the “amount of warming that a gas will cause in the next 100 years, compared to the same volume of carbon dioxide."
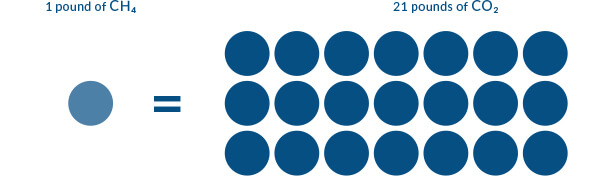
This graphic uses methane (CH4) to illustrate how Global Warming Potential is conveyed.
In 100 years, a pound of methane (single circle) will have the same global warming effect as approximately 21 pounds of carbon dioxide (21 circles). This puts the Global Warming Potential of methane at 21 in this comparison to carbon dioxide. [The range of GWP values typically referenced for methane over 100 years is between 21 and 38.] For more information about Global Warming Potential see this website http://www3.epa.gov/climatechange/ghgemissions/gwps.html
What contributes to Global Warming Potential?

Global Warming Potential for each gas in our atmosphere depends on two things:
- how much infrared (IR) light the gas absorbs and
- how long the gas stays in the atmosphere.
1) Absorbing IR
When infrared (IR) light strikes a bond, that bond absorbs the light and causes the bond to stretch. Some bonds can absorb more energy from the IR light than others. Compare this to what you know about the water absorbancy of cotton fibers versus polyester fibers. Cotton fibers naturally absorb more water than polyester fibers just like some molecular bonds can absorb more IR light than others.
2) How long gases remain in the atmosphere
Some global warming gases are more stable in the environment than others. A good analogy would be different degradation rates of paper and plastic when exposed to the outdoor environment. We expect both paper and plastic to deteriorate with environmental exposure, however we generally expect that paper will degrade faster than plastic when left outdoors. Similarly, some gases degrade faster than others. Global warming gas molecules in the atmosphere degrade because sunlight and other chemicals in the air break their bonds, or they go out into space, or they return to the surface of the earth. How long a gas stays in the atmosphere is called its residence time.
Global Warming Potential
The table below shows common greenhouse gases, their GWP, residence time in years, relative ability to absorb IR and common sources. The sources of GHGs related to textile production have been highlighted here.
| Gas | Global Warming Potential | Residence TIme (year) | Relative Ability to Absorb Infrared | Common Source |
|---|---|---|---|---|
| Carbon Dioxide CO2 |
1 | 50-200 | 1 | Fossil fuel burning |
| Methane CH4 |
21 | 12 | 43 | Agriculture, refining |
| Nitrous Oxide N4O |
310 | 120 | 250 | Agriculture, nylon |
| Chlorofluoro- carbons CFCs |
7,100 | 195 | 19,000 | Refrigerants |
| Perfluoro- carbons PFCs |
~9,000 | 2,600-4,000 | 21,000 | Stain resistant fabric finishes |
Notice the importance of these gases to the fabric and textile industries. Besides PFCs and N2O, the manufacture of all textiles requires energy to produce electricity and heat. Nearly all of the energy used in the industry is from fossil fuels. Methane is released in petroleum refining and fracking operations. In addition to energy, petroleum provides the raw materials for production of many textiles.
The residence time of several greenhouse gases are shown in the third column of this table. All of the years that these gases are in the atmosphere they are acting as global warming gases, absorbing IR light and contributing to increased temperatures.
Notice that carbon dioxide will stay in the atmosphere much longer than methane, and that PFCs, which are used on stain resistant fabrics, have very, very long residence times in the atmosphere. N2O is a compound that comes from the manufacture of nylon, and is important in agricultural based textiles and dyes. It also stays in the atmosphere a relatively long time of 120 years.
Finally, this table above shows the Global Warming Potential (GWP) of the GHGs listed. Remember that GWP is the amount of warming that a gas will cause in the next 100 years, compared to the same volume of carbon dioxide. It is important to point out that the global warming potential is a combination of the years the gas will reside in the atmosphere and the ability of the gas to absorb infrared light energy. Note for example, that the residence time of methane, CH4, is shorter than that for carbon dioxide, but it has a higher GWP because it strongly absorbs IR radiation.
Please view this video as a review of the content related to Global Warming Potential.
In the Classroom
Global Warming Potential
Global Warming Potential - 5.2.3 - PowerPoint PresentationMore Information and Resources
Global Warming Potential
- Pacific Institute for Climate Solutions Examples of Global Warming (7:09 minutes) http://www.youtube.com/watch?v=VttL3ZYQpy4&feature=youtu.be
- EPA Climate Change website http://www3.epa.gov/climatechange/
