Chapter 5.2.1 / Atmosphere & Greenhouse Gases
Atmosphere & Greenhouse Gases
The natural greenhouse effect is necessary for the temperature of earth to support current life systems. The enhanced greenhouse effect caused by a greater buildup of carbon dioxide and methane (and other greenhouse gases) leads to less radiated heat leaving the atmosphere, resulting in warmer global temperatures. Scientists are confident that human burning of fossil fuels is a major contributor to the enhanced greenhouse effect.
World population is increasing, industrialization is expanding globally, income levels are rising in newly industrialized countries spurring greater levels of consumption. These realities are contributing to even more rapid increases in greenhouse gases.
Professional Development for Educators
Atmosphere & Greenhouse Gases
The Earth’s energy balance is regulated by the atmosphere. The atmosphere is made up of gases. These gases regulate energy transmission through the atmosphere. This is where greenhouse gases come into the picture.
[As an educator, be aware that some students believe that our atmosphere is only the clouds and above the clouds. It is essential for them to know that the atmosphere starts at the surface of the Earth and extends more than 300 miles outward from the surface of the Earth.]
The illustrations below show the Earth's atmosphere visually and then diagramatically.
Image Source: NASA http://earthobservatory.nasa.gov/IOTD/view.php?id=7373
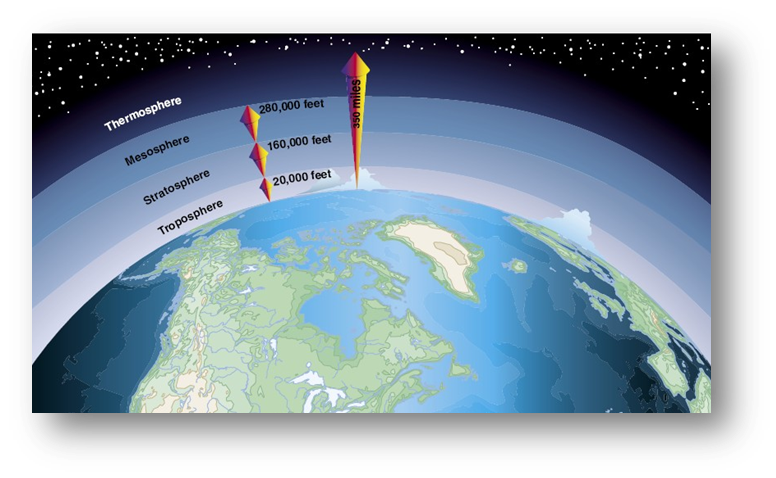
Source: http://www.groundinstructor.com/mod/page/view.php?id=970
Understanding the Science of the Greenhouse Effect
In order to understand the basic science of the greenhouse effect, two concepts must be explained: the science of light and the structure of the gases in our atmosphere.
Understanding the Science of Light

Let’s begin with light. We’ll use “light” here to refer to all electromagnetic radiation (EMR) which includes many kinds of light such as ultraviolet light, infrared light, X-rays, microwaves and many more. The illustration above shows the visible light spectrum.
Light can be understood by three basic characteristics: wavelength, frequency and energy.
Under In the Classroom below there is a classroom activity that provides an easy way for students to remember these three basic characteristics.
Electromagnetic Radiation
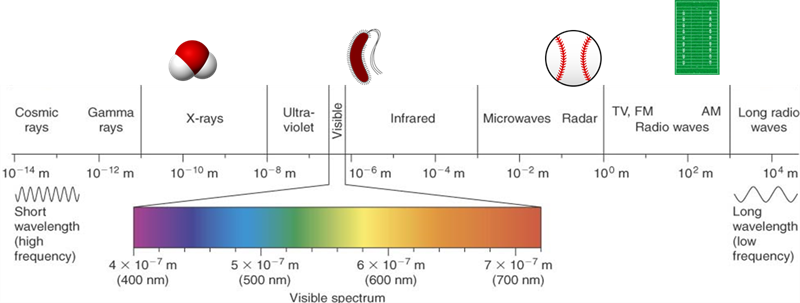
The whole range of EMR from the very short, very energetic cosmic rays to the very long, low energy radio waves acts the same way: Each wavelength has a frequency and energy associated with it.
Visible light spectrum is a small portion of the total type of electromagnetic radiation (EMR). To the left on this illustration there are shorter (invisible) ultraviolet wavelengths with higher frequency and more energy. The longer infrared wavelengths to the right of visible spectrum have lower frequency with less energy.
Light (or EMR) has an incredible variation in wavelength. Very small wavelength and very energetic light like X-rays are very high energy. We know they are high in energy because X-rays can go right through us to make the images of our bones we call “X-rays”! On the other hand, radio and TV waves are huge – often meters long, and of very low energy.
The energy of light (EMR) is fundamentally related to how the Earth's atmosphere traps heat, or the greenhouse effect. Under In the Classroom below there is a classroom activity in the form of a POGIL, which stands for Process Oriented Guided Inquiry Learning. This POGIL provides students the opportunity to examine how the energy in sunlight interacts with the atmosphere to trap heat.
In the Classroom
Atmosphere & Greenhouse Gases
ACTIVITY: Demonstrating the wavelength, frequency and energy of light Wavelength Activity - Video Demonstration ACTIVITY (POGIL): The Earth's GreenhouseYou can learn more about POGIL at: https://pogil.org/about
What is POGIL? from The POGIL Project on Vimeo.
More Information and Resources
Atmosphere & Greenhouse Gases
- This link to a website called EXPLAINTHATSTUFF! provides an overview of the natural greenhouse effect http://www.explainthatstuff.com/globalwarmingforkids.html
- The Department of Geosciences at Georgia State University has published a set of labs that may be helpful. Of particular interest are the following:
- Lab 2 Stratospheric Ozone http://sites.gsu.edu/geog1112/lab-4/
- Lab 3 The Troposphere http://sites.gsu.edu/geog1112/the-troposphere/
- NASA A Year in the Life of Earth’s CO2 (3:10 minutes) https://www.youtube.com/watch?feature=player_embedded&v=x1SgmFa0r04
- An overview of the Earth's atmosphere http://www.groundinstructor.com/mod/page/view.php?id=970
